More Information
Submitted: May 03, 2023 | Approved: June 15, 2023 | Published: June 16, 2023
How to cite this article: Kumari V, Sharma G, Mohil, Kanwar SS. Human Monkeypox Virus Severity. Ann Proteom Bioinform. 2023; 7: 014-020.
DOI: 10.29328/journal.apb.1001021
Copyright License: © 2023 Kumari V, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Keywords: Monkeypox; Epidemiology; Zoonotic disease; Prevention; Transmission
Abbreviations: ACC: Accuracy; BiDNNs: Bidirectional Deep Neural Networks; C-index: Concordance Index; CNV: Copy Number Variation
Human Monkeypox Virus Severity
Vandna Kumari1, Gaurav Sharma2, Mohil3 and Shamsher S Kanwar4*
1,3,4Department of Biotechnology, Himachal Pradesh University, Summer Hill, Shimla, 171 005. India
2Department of Microbiology, Shoolini Institute of Life Sciences and Business Management Sciences, Solan, 173212. India
*Address for Correspondence: Shamsher S Kanwar, Department of Biotechnology, Himachal Pradesh University, Summer Hill, Shimla, 171 005. India, Email: [email protected]
Monkeypox is a sylvatic zoonotic sickness that initially affected tropical rainforest areas in the west and vital Africa earlier than spreading to other places. Its miles carried with the aid of the Monkeypox virus member of orthopoxvirus circle of relatives. The clinical features of the infection resembles to smallpox. The primary animal to human transmission is the cause of most people of human Monkeypox ailments. Severe rodent species and non-human primates function hosts for the virus. Transmission can manifest with infected people, animals or objects into contact with bodily fluids, sores on the skin or inner mucosal surfaces just like the mouth or throat, or breathing droplets. The incubation length for Monkeypox usually lasts 6 to 13 days, even though it could last as long as 21 days. The ailment is often self-proscribing, with signs and symptoms generally clearing up on their own inside 14 to 21 days. Signs can range from moderate to extreme, and lesions may be itchy or painful. Due to the discontinuation of recurring smallpox vaccination which supplied some pass- safety in past, populations have become more liable to Monkey pox. The outbreak of Monkeypox virus due to the fact that may additionally, 2022 has created a worldwide risk of the virus. In the present review, Monkeypox epidemiology, severity, therapeutics, vaccination and its transmission to non-endemic countries has been considered. Special care and guidelines may help in the containing in the spread of the infections to the non endemic countries.
Monkeypox is a viral zoonotic disorder in most cases discovered in tropical rainforest regions of imperative and West Africa, with sporadic exportations to different places. In non-endemic nations, Monkeypox outbreak has persisted in 2022 [1]. The human Monkeypox virus (MPXV) is double-stranded DNA virus belonging to the family Poxviridae's Orthopoxvirus genus. West African and relevant African MPXV clades have been given genetic descriptions. The first instances of Monkeypox out of doors of Africa had been recognized inside the USA in 2003 [2]. Human-to-human transmission has been shown instances from UK in 2018 [3] and additionally inside the current multi-united states outbreak [3]. MPXV consists of complex double stranded DNA viruses which can be distinguished by means of their replication within the cytoplasm of vertebrate or invertebrate cells [4]. Transmission electron microscopy, PCR, immuno-fluorescence and ELISA are the techniques which may be used for the identification of MPXV [5]. RT-PCR (actual-time reverse transcription PCR) [6] have been used to perceive the virus in pustule swabs. The endorsed laboratory take a look at for Monkeypox with polymerase chain reaction (PCR), which detects DNA virus. The great diagnostic samples come immediately from the rash and can be skin, fluid, crusts or in some instances biopsy methods for detecting antigens and antibodies that do not differentiate among orthopoxviruses may not be useful. In the Democratic Republic of the Congo, an infant with smallpox-like outbreaks was located to have human Monkeypox in 1970 [7]. The orthopoxvirus, which is known to provide epidemics in restricted monkeys, became recognized from skin lesions on an imported macaque in a Danish laboratory in 1958 [8]. Regardless of the reality that antibodies to orthopoxvirus had been determined in a number of rodent and primate species in West Africa, only two times MPXV have been isolated from sylvatic animals, whereas the appropriate reservoir of the virus remains unknown [8,9]. Macular- papular, vesicular, pustular and eventually crusted scab lesions frequently follows a one to four day fever prodrome with headache and fatigue [10]. At each stage, lesions stays between one and three days and develop simultaneously. In comparison to smallpox, lymphadenopathy can seem before or at some point of the rash. The ailment's severity stages are from moderate to intense and deadly [10] headaches have been pronounced, including diarrhea and vomiting, corneal scarring and conjunctivitis, broncho-pneumonia, encephalitis, and sepsis [8].
An average long-time period trouble is everlasting pitted scarring caused by bacterial super-infection [8]. Pregnant ladies have experienced extra severe disease and miscarriages [11,12]. Smallpox vaccine may be used against the Monkeypox with an effectiveness of 85%. The frequency and severity of signs and symptoms reduces significantly.
The frequency and severity of signs and symptoms are significantly reduced by residual immunity from previous vaccination [13,14]. In unvaccinated humans, mentioned case fatality fees (CFR) range from 0% to 11% [15]. Immuno-compromised human beings, including those with untreated HIV infections, are predisposed to more critical ailment and have high possibilities of life loss [16]. Monkeypox outbreaks are maximum common in communities that hunt, kill, cope with and devour bush meat [17-19] primarily advent through lesion fabric and respiration droplet [20,21]. The incubation period lasts among 7 and 14 days [22]. From the time the rash appears to four weeks later, patients are considered infectious man or woman-to-character transmission can appear via respiratory droplets, the placenta, or direct contact with skin fomites or abrasions [23-25].
The fundamental clades of MPXV are the Congo Basin (CB) strain and the West African (WA) strain. The CB strain is related to accelerated morbidity, mortality, and transmission from person to man or woman [26]. The most preferred methods for confirming MPXV contamination are virus isolation, electron microscopy, and PCR [27,28] but, in far off regions, most people of cases are clinically diagnosed. In the course of Monkeypox outbreaks, chickenpox is frequently misdiagnosed as Monkeypox [29,30]. A small number of compounds developed for smallpox can be broadly tested for treatment efficacy with Monkeypox [31]. In endemic regions, instances of Monkeypox are currently being controlled supportively. The use of the vaccinia vaccine to stop the spread of Monkeypox in endemic areas has gotten little interest due to the fact a fatality passed off after vaccination of an HIV- effective character, this approach of preventative measures is rarely utilized in endemic settings [32].
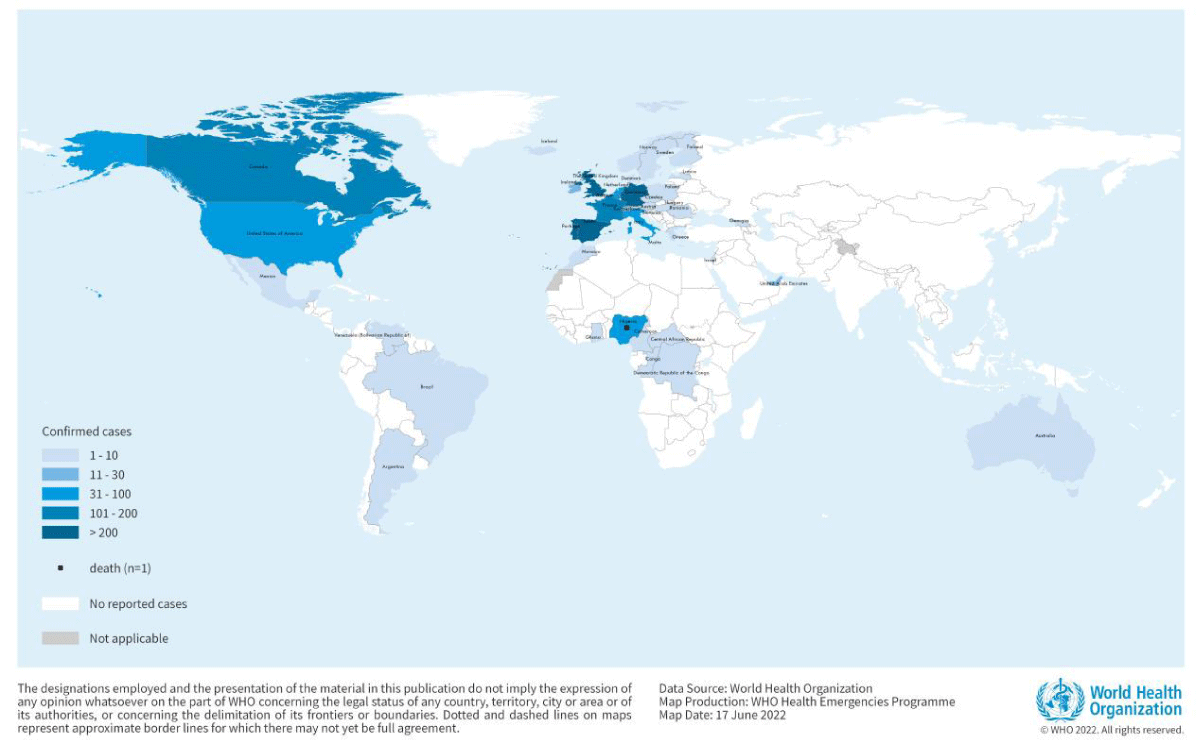
Monkeypox has currently been mentioned in Nigerians touring to Israel in September 2018, United kingdom in September 2018, December 2019, May 2021 and May 2022, Singapore in May 2019, and United states in July and November 2021. Multiple cases of Monkeypox were located in many non-endemic international locations in May 2022. On May 16, 2022, a multi-country MPXV outbreak began that was consider that spread to the United Kingdom (United Kingdom), the Europe, Asia, the Americas, and Australia. As of June 22nd, 29 international locations and areas in Europe had recorded 2746 instances of Monkey pox. In 2022, the European union centre for disorder prevention and management mentioned 3,417 showed instances of Monkeypox 58 international locations and has been declared the disease to be pandemic (WHN). Over several continents, the variety of instances are growing at an increasing rate each month (Figure 1).
Figure 1: Geographic distribution of Monkey pox cases reported to or identified by WHO from official public sources between January 1, 2022 and September 30, 2022 (Source: World Health Organization).
MPXV severity
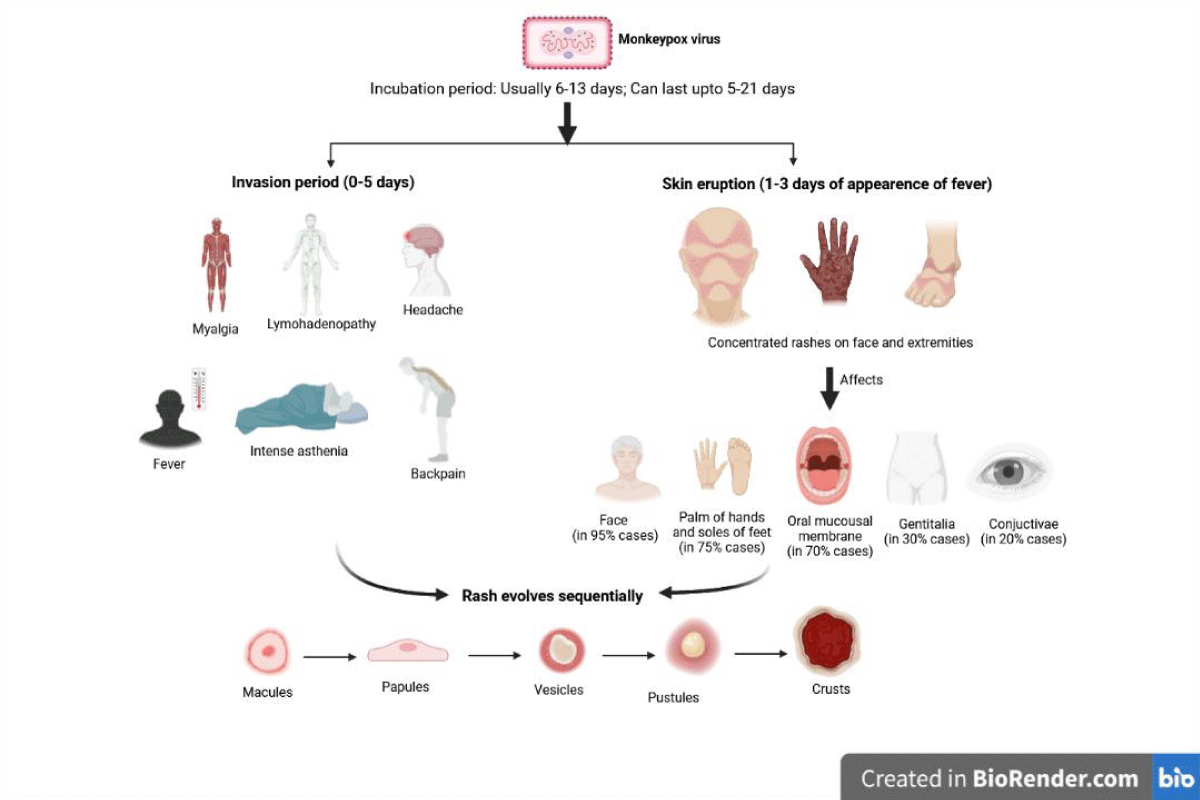
Monkeypox is characterized through an in depth feature rash, swollen lymph nodes and fever. Monkeypox is distinguished from different illnesses such as measles, scabies, syphilis, bacterial pores and skin infections, chickenpox and medicine-related hypersensitive reactions. Monkeypox has an incubation period of 6- 21 days. The infection is broken down into two stages: the invasion phase, which lasts 0 to 5 days and is characterized by fever, a lot of headaches, lymphadenopathy (swelling of the lymph nodes), lower back pain, a lot of asthenia (loss of energy), myalgia (muscle aches), and skin eruption phase. Monkeypox can be distinguished from other infections, such as measles, chickenpox, and smallpox, by their lymphadenopathy. Within 1-3 days of the onset of the fever, the skin eruption typically starts [33-35]. Instead of the trunk, the rash typically affects the face and extremities. The cheeks, the bottoms of the toes, and the palms of the hands are typically affected (in 75% of cases). Conjunctivae (20%), the cornea (70%), oral mucous membranes (70%), and genitalia (30%) are also impacted. The rash progresses from macules, which are flat-based total lesions, to papules, which are scarcely raised company lesions, vesicles, which are clear fluid-filled lesions, pustules, which are yellow fluid-filled lesions, and crusts that dry up and break off [36,37].The variety of lesions degrees from a few hundred to lots (Figure 2). The progression of lesions includes macules (flat-base lesions), papules (raised, painful lesions), vesicles (packs of clear fluid), pustules (filled with pus), and then scabs or crusts. In reported occurrences, the percentage of victims who passed away ranged from 0% to 11%, with younger children making up the majority of the fatalities. In extreme cases, lesions can merge and slough off big sections of skin.
Figure 2: MPXV infection phases and symptoms.
A self-proscribing infection, Monkeypox often has symptoms that last two to four weeks [38]. Monkeypox case death rates have historically ranged from 0% to 11% in the well-known population, with small children being more susceptible. The case fatality ratio has been circling at 3% - 6% recently [39]. Immune deficits may also contribute to more serious outcomes. Despite the fact that the smallpox vaccine proved effective, people under the age of 40 to 50 (depending on the USA) may now also be more susceptible to Monkeypox due to the worldwide end of smallpox immunisation campaigns after the disease was eradicated [40,41]. Secondary infections, sepsis, bronchopneumonia, encephalitis, and corneal infection result in misplaced imaginative and prescient are in all likelihood aspect outcomes of Monkeypox [42]. It is far unknown how great asymptomatic contamination can be but asymptomatic Orthopoxvirus flow in Cameroon was located to be very likely [43].
Therapeutics and vaccination
Patients with Monkeypox require supportive care based on their signs and symptoms (Table 1).
| Table 1: Drugs approved for monkeypox with theirmode of action. | |||
| Drug | Mode of action | Major drug interaction | Common adverse effects |
| Tecovirimat (Approved in May 2022) | Orthopoxvirus VP37 envelope wrapping protein inhibitor | Repaglinide (hypoglycemia),Midazolam (decreased effectiveness of midazolam) |
Headache, nausea, abdominal pain, vomiting. Infusion-site reactions may occur with IV form |
| Cidofovir | Undergoes cellular phosphorylation, then selectively inhibits orthopoxvirus DNA polymerase-mediated viral DNA synthesis | Probenecid, agents of nephrotoxic potential | Decreased serum bicarbonate, proteinuria, neutropenia, infection, hypotony of eye, iritis, uveitis, nephrotoxicity, fever. |
| Vaccinia immune globulin | Antibodies obtained from pooled human plasma of individuals immunized with the smallpox vaccine provide passive immunity | Contains maltose: may result in elevated glucose readings that can lead to untreated hypoglycemia or inappropriate insulin administration; may impair efficacy of live attenuated virus vaccines: revaccination may be necessary; may interfere with some serological tests | Headache, nausea, rigors, dizziness |
| Brincidofovir | Phosphorylated to active metabolite, cidofovir diphosphate, which selectively inhibits orthopoxvirus DNA polymerase-mediated viral DNA synthesis | OATP1B1 and 1B3 inhibitors increase Brincidofovir exposure which may increase Brincidofovir -associated adverse reactions. Consider alternative medication that are not OATP1B1 or 1B3 inhibitors | Diarrhea, nausea, vomiting, and abdominal pain |
Several compounds that have viable effectiveness against Monkeypox contamination have been testified. For the treatment of smallpox, two oral medicinal drugs, brincidofovir and tecovirimat, have acquired approval and feature shown efficacy in animal research against Monkeypox [44-46]. The EU drugs employer (EMA) licenced secondary tecovirimat, developed for smallpox, for Monkeypox in 2022 based totally on information from human and animal research but tecovirimat have to preferably be determined for utilization for patient treatment.
Numerous observational research have shown that smallpox vaccination's effectiveness in keeping off Monkeypox is roughly 85% [47].
As an end result, previous smallpox vaccination may be used to milder infection. A latest vaccination based on attenuated vaccinia virus was accepted in 2019 to prevent Monkeypox [48].
This is a dose vaccine with constrained availability. Because of the go-safety afforded with the aid of the immune response to ortho-poxviruses, Monkeypox and smallpox vaccines are developed in formulations based totally on the vaccinia virus.
Transmission of MPXV
Transmission from animal to human (zoonotic) can show up through direct touch with infected animal's blood, cutaneous or mucosal lesions or bodily fluids [49].
Evidence indicates several animals were found to have the Monkeypox virus in Africa primarily, which include various monkey species, tree squirrels, rope squirrels, dormice, Gambian poached rats and others. Rodents are the most probable natural reservoir for Monkey pox, though this has no longer yet been determined. Consuming undercooked meat and different animal products from infected animals may additionally pose a chance. People who live in or near forested regions can be exposed to infected animals in an indirect or low-stage way [50]. Human to human transmission occurs through physical touch, breathing secretions or infected droplets. This will be due to a decline in immunity throughout all communities due to the cessation of smallpox vaccination [51]. Transmission can also occur from mother to foetus through the placenta which could bring congenital Monkeypox or during the close touch during and after delivery [52].
Although close physical interaction is a acknow-ledged reason for transmission, it's far doubtful if sexual contact can transmit Monkeypox [53]. For a better understanding of this threat, much greater research is needed.
In non-endemic international locations, a Monkeypox outbreak has continued from May 2022 [53]. Human Monkeypox virus (MPXV) is a double stranded DNA virus of the Orthopoxvirus genus of the Poxviridae family. MPXV consists of complex double stranded DNA virus this is outstanding by means of its replication in a vertebrate’s or an invertebrate’s cytoplasm. The encouraged laboratory test for Monkeypox is polymerase chain response (PCR) detection of virus DNA. People who have impaired immune structures, inclusive of those with untreated HIV infections are extra susceptible for the infection. Fever, a huge unique rash, and enlarged lymph nodes are the hallmarks of Monkey pox. Chickenpox, measles, bacterial skin illnesses, scabies, syphilis and medicinal drug associated hypersensitive reactions ought to all be separated from Monkey pox. MPXV has an incubation period of 5-21 days. Two oral medicines, tecovirimat and brincidofovir had been accepted for smallpox treatment and have proven effectiveness for Monkeypox in trials on animals. One of the extra recent vaccinations that has been created is licensed Monkeypox scientific remedy must be absolutely based on lesion signs and symptoms, taken care of headaches, and prevent long-term bacterial infections.
Increased public knowledge of danger elements and training on feasible measures to lessen viral exposure are the cornerstones of Monkeypox prevention. A systematic evaluation of the viability and suitability of vaccination for the prevention and manage of Monkeypox is now being carried out. A few countries have or are developing policies to offer vaccines to the persons who can be greater danger together with laboratory personnel, fast reaction teams and medical examiners. To include a pandemic, surveillance and quick case identity are important. The principle threat factor for Monkeypox virus infection throughout MPXV epidemics is intimate touch with infected people. The threat of contamination is higher for family of contributors and healthcare group of workers. Fitness specialists ought to follow the endorsed contamination manage processes even as dealing with patients with a Monkeypox virus contamination that has been suspected or showed or whilst specimens from such patients. In compliance with WHO guidelines for the secure transfer of infectious substances, patient specimens should be triple packaged. Much intensive research is required to identify the infection's beginning possibilities. Elevating public awareness and educating healthcare experts are critical to the prevention and control of Human Monkeypox. Most human Monkeypox infection effects from a primary animal-to-human transmission. Animal meat or parts need to be nicely cooked earlier than intake, and contact with sick or deceased animals should be avoided. Close contact with infected individuals should be avoided. Gloves and other private safety gears must be used while taking care of the infected persons in clinical facilities as well as at home places. Animals saved in captivity who might have Monkeypox should be quarantined right away and saved break free other animals. Animals that may have interacted with other infected animal need to be constrained, treated with regular safety measures and monitored for symptoms of Monkeypox for 30 days. Residents and traffic to endemic international locations must stay far away from unwell or dead rodents, marsupials or primates that may be carrying the MPXV and ought to abstain from eating or managing bush meat. According to WHO pointers, the importance of hand hygiene with water and soap or an alcohol-based totally sanitizer must be emphasized, especially during the pores and skin eruption length of disorder (with the vesicular rash), isolation or quarantine of cases. Some countries including Belgium, have brought a mandatory 21 days quarantine for Monkeypox patients as instances spread globally. As a few specialists suggest, trying out and containing the infection are the right measures to be carried out [53].
Human Monkeypox virus (MPXV), zoonotic disease is a double-stranded DNA virus belonging to the family Poxviridae's orthopoxvirus genus. In non-endemic nations, Monkeypox outbreak has persisted in 2022. Transmission electron microscopy, PCR, immuno-fluorescence and ELISA are the techniques which may be used for the identification of MPXV. A small number of compounds developed for smallpox can be broadly tested for treatment efficacy with Monkey pox. As per the report of WHN and European Union centre for disorder prevention and management Monkeypox was declared the disease to be pandemic in 2022. Monkeypox is characterized through an in depth feature rash, swollen lymph nodes and fever. Monkeypox is distinguished from different illnesses such as measles, scabies, syphilis, bacterial pores, skin infections, chickenpox and medicine-related hypersensitive reactions. Monkeypox has an incubation period of 5- 21 days. Several compounds that have viable effectiveness against Monkeypox contamination have been testified. For the treatment of smallpox, two oral medicinal drugs, brincidofovir and tecovirimat, have acquired approval and feature shown efficacy in animal research against Monkey pox. Smallpox vaccine may also be used against the Monkeypox with an effectiveness of 85 percent. Human to human transmission occurs through physical touch, breathing secretions or infected droplets. Transmission can also occur from mother to foetus through the placenta which could bring congenital Monkeypox or during the close touch during and after delivery. Although close physical interaction is an acknowledged reason for transmission, it is far doubtful if sexual contact can transmit Monkey pox. For a better understanding of this threat, much greater research is needed. To include a pandemic, surveillance and quick case identity are important. In compliance with WHO guidelines for the secure transfer of infectious substances, patient specimens should be triple packaged. As suggested by few specialists, trying out and containing the infection are the right measures to be carried out for containing the MPXV infections. Elevating public awareness and educating healthcare experts are critical to the prevention and control of Human Monkeypox.
The authors are thankful to DST and DBT, Ministry of Science & Technology, New-Delhi for continuous financial support to the Bioinformatics Centre, Himachal Pradesh University, Shimla (India).
- Antinori A, Mazzotta V, Vita S, Carletti F, Tacconi D, Lapini LE, D'Abramo A, Cicalini S, Lapa D, Pittalis S, Puro V, Rivano Capparuccia M, Giombini E, Gruber CEM, Garbuglia AR, Marani A, Vairo F, Girardi E, Vaia F, Nicastri E; INMI Monkeypox Group. Epidemiological, clinical and virological characteristics of four cases of monkeypox support transmission through sexual contact, Italy, May 2022. Euro Surveill. 2022 Jun;27(22):2200421. doi: 10.2807/1560-7917.ES.2022.27.22.2200421. PMID: 35656836; PMCID: PMC9164671.
- Reynolds MG, Yorita KL, Kuehnert MJ, Davidson WB, Huhn GD, Holman RC, Damon IK. Clinical manifestations of human monkeypox influenced by route of infection. J Infect Dis. 2006 Sep 15;194(6):773-80. doi: 10.1086/505880. Epub 2006 Aug 8. PMID: 16941343.
- Vaughan A, Aarons E, Astbury J, Brooks T, Chand M, Flegg P, Hardman A, Harper N, Jarvis R, Mawdsley S, McGivern M, Morgan D, Morris G, Nixon G, O'Connor C, Palmer R, Phin N, Price DA, Russell K, Said B, Schmid ML, Vivancos R, Walsh A, Welfare W, Wilburn J, Dunning J. Human-to-Human Transmission of Monkeypox Virus, United Kingdom, October 2018. Emerg Infect Dis. 2020 Apr;26(4):782-785. doi: 10.3201/eid2604.191164. Epub 2020 Apr 17. PMID: 32023204; PMCID: PMC7101111.
- Mahase E. Monkeypox: What do we know about the outbreaks in Europe and North America? BMJ. 2022 May 20;377:o1274. doi: 10.1136/bmj.o1274. PMID: 35595274.
- Moss B. Poxviridae: The viruses and their replication. In “Fields Virology” (DM. Knipe, Howley PM. Eds. Lippincott, Williams & Wilkins, Philadelphia. 2: 2849–2883.
- Erez N, Achdout H, Milrot E, Schwartz Y, Wiener-Well Y, Paran N, Politi B, Tamir H, Israely T, Weiss S, Beth-Din A, Shifman O, Israeli O, Yitzhaki S, Shapira SC, Melamed S, Schwartz E. Diagnosis of Imported Monkeypox, Israel, 2018. Emerg Infect Dis. 2019 May;25(5):980-983. doi: 10.3201/eid2505.190076. Epub 2019 May 17. PMID: 30848724; PMCID: PMC6478227.
- Costello V, Sowash M, Gaur A, Cardis M, Pasieka H, Wortmann G, Ramdeen S. Imported Monkeypox from International Traveler, Maryland, USA, 2021. Emerg Infect Dis. 2022 May;28(5):1002-1005. doi: 10.3201/eid2805.220292. Epub 2022 Mar 9. PMID: 35263559; PMCID: PMC9045429.
- Marennikova SS, Seluhina EM, Mal'ceva NN, Ladnyj ID. Poxviruses isolated from clinically ill and asymptomatically infected monkeys and a chimpanzee. Bull World Health Organ. 1972;46(5):613-20. PMID: 4340220; PMCID: PMC2480788.
- Magnus P, Andersen EK, Petersen KB, Birch-Andersen A. A Pox-like Disease in Cynomolgus Monkeys. Acta Pathologica Microbiologica Scandinavica. 1959; 46(2):156–76. https://doi.org/10.1111/j.1699-0463.1959.tb00328.x
- Doty JB, Malekani JM, Kalemba LN, Stanley WT, Monroe BP, Nakazawa YU, Mauldin MR, Bakambana TL, Liyandja Dja Liyandja T, Braden ZH, Wallace RM, Malekani DV, McCollum AM, Gallardo-Romero N, Kondas A, Peterson AT, Osorio JE, Rocke TE, Karem KL, Emerson GL, Carroll DS. Assessing Monkeypox Virus Prevalence in Small Mammals at the Human-Animal Interface in the Democratic Republic of the Congo. Viruses. 2017 Oct 3;9(10):283. doi: 10.3390/v9100283. PMID: 28972544; PMCID: PMC5691634.
- Hutin YJ, Williams RJ, Malfait P, Pebody R, Loparev VN, Ropp SL, Rodriguez M, Knight JC, Tshioko FK, Khan AS, Szczeniowski MV, Esposito JJ. Outbreak of human monkeypox, Democratic Republic of Congo, 1996 to 1997. Emerg Infect Dis. 2001 May-Jun;7(3):434-8. doi: 10.3201/eid0703.010311. PMID: 11384521; PMCID: PMC2631782.
- Systematic review human Monkey pox outbreaks. PLOS Neglected Tropical Diseases https://doi.org/10.1371/journal.pntd.0007791 October 16, 2019 16 / 20
- Khodakevich L, Jezek Z, Messinger D. Monkeypox virus: ecology and public health significance. Bull World Health Organ. 1988;66(6):747-52. PMID: 2853010; PMCID: PMC2491157.
- Jezek Z, Grab B, Szczeniowski M, Paluku KM, Mutombo M. Clinico-epidemiological features of monkeypox patients with an animal or human source of infection. Bull World Health Organ. 1988;66(4):459-64. PMID: 2844428; PMCID: PMC2491168.
- Reynolds MG, Carroll DS, Olson VA, Hughes C, Galley J, Likos A, Montgomery JM, Suu-Ire R, Kwasi MO, Jeffrey Root J, Braden Z, Abel J, Clemmons C, Regnery R, Karem K, Damon IK. A silent enzootic of an orthopoxvirus in Ghana, West Africa: evidence for multi-species involvement in the absence of widespread human disease. Am J Trop Med Hyg. 2010 Apr;82(4):746-54. doi: 10.4269/ajtmh.2010.09-0716. PMID: 20348530; PMCID: PMC2844556.
- Jezek Z, Szczeniowski M, Paluku KM, Mutombo M. Human monkeypox: clinical features of 282 patients. J Infect Dis. 1987 Aug;156(2):293-8. doi: 10.1093/infdis/156.2.293. PMID: 3036967.
- Mbala PK, Huggins JW, Riu-Rovira T, Ahuka SM, Mulembakani P, Rimoin AW, Martin JW, Muyembe JT. Maternal and Fetal Outcomes Among Pregnant Women With Human Monkeypox Infection in the Democratic Republic of Congo. J Infect Dis. 2017 Oct 17;216(7):824-828. doi: 10.1093/infdis/jix260. PMID: 29029147.
- Withers MR, Kingebeni PM, Muyembe JJT, Martin J, Riu-Rovira T, Huggins J. Outcome of fourpregnancies in congolese women with Monkey pox infection. American Journal of Tropical Medicineand Hygiene. 2011; (1):397. PMID: 71043487.
- Kisalu NK, Mokili JL. Toward Understanding the Outcomes of Monkeypox Infection in Human Pregnancy. J Infect Dis. 2017 Oct 17;216(7):795-797. doi: 10.1093/infdis/jix342. PMID: 29029238; PMCID: PMC6279131.
- Fine PE, Jezek Z, Grab B, Dixon H. The transmission potential of monkeypox virus in human populations. Int J Epidemiol. 1988 Sep;17(3):643-50. doi: 10.1093/ije/17.3.643. PMID: 2850277.
- Heymann DL, Szczeniowski M, Esteves K. Re-emergence of monkeypox in Africa: a review of the past six years. Br Med Bull. 1998;54(3):693-702. doi: 10.1093/oxfordjournals.bmb.a011720. PMID: 10326294.
- Meyer H, Perrichot M, Stemmler M, Emmerich P, Schmitz H, Varaine F, Shungu R, Tshioko F, Formenty P. Outbreaks of disease suspected of being due to human monkeypox virus infection in the Democratic Republic of Congo in 2001. J Clin Microbiol. 2002 Aug;40(8):2919-21. doi: 10.1128/JCM.40.8.2919-2921.2002. PMID: 12149352; PMCID: PMC120683.
- Mutombo M, Arita I, Jezek Z. Human monkeypox transmitted by a chimpanzee in a tropical rain-forest area of Zaire. Lancet. 1983 Apr 2;1(8327):735-7. doi: 10.1016/s0140-6736(83)92027-5. PMID: 6132084; PMCID: PMC9534202.
- Reynolds MG, Yorita KL, Kuehnert MJ, Davidson WB, Huhn GD, Holman RC, Damon IK. Clinical manifestations of human monkeypox influenced by route of infection. J Infect Dis. 2006 Sep 15;194(6):773-80. doi: 10.1086/505880. Epub 2006 Aug 8. PMID: 16941343.
- Jezek Z, Grab B, Paluku KM, Szczeniowski MV. Human monkeypox: disease pattern, incidence and attack rates in a rural area of northern Zaire. Trop Geogr Med. 1988 Apr;40(2):73-83. PMID: 2841783.
- Jezek Z, Grab B, Szczeniowski MV, Paluku KM, Mutombo M. Human Monkey pox: secondary attackrates. Bulletin of the World Health Organization. 1988; 66(4):465–70. PMID: 2844429.
- Parker S, Nuara A, Buller RM, Schultz DA. Human Monkey pox: an emerging zoonotic disease. FutureMicrobiology. 2007; 2(1):17–34. https://doi.org/10.2217/17460913.2.1.17 PMID: 17661673.
- Likos AM, Sammons SA, Olson VA, Frace AM, Li Y, Olsen-Rasmussen M, Davidson W, Galloway R, Khristova ML, Reynolds MG, Zhao H, Carroll DS, Curns A, Formenty P, Esposito JJ, Regnery RL, Damon IK. A tale of two clades: monkeypox viruses. J Gen Virol. 2005 Oct;86(Pt 10):2661-2672. doi: 10.1099/vir.0.81215-0. PMID: 16186219.
- Panning M, Asper M, Kramme S, Schmitz H, Drosten C. Rapid detection and differentiation of human pathogenic orthopox viruses by a fluorescence resonance energy transfer real-time PCR assay. Clin Chem. 2004 Apr;50(4):702-8. doi: 10.1373/clinchem.2003.026781. Epub 2004 Feb 12. PMID: 14962998.
- Neubauer H, Reischl U, Ropp S, Esposito JJ, Wolf H, Meyer H. Specific detection of monkeypox virus by polymerase chain reaction. J Virol Methods. 1998 Oct;74(2):201-7. doi: 10.1016/s0166-0934(98)00099-8. PMID: 9779620.
- Li Y, Olson VA, Laue T, Laker MT, Damon IK. Detection of monkeypox virus with real-time PCR assays. J Clin Virol. 2006 Jul;36(3):194-203. doi: 10.1016/j.jcv.2006.03.012. Epub 2006 May 30. PMID: 16731033; PMCID: PMC9628957.
- Jezek Z, Szczeniowski M, Paluku KM, Mutombo M, Grab B. Human monkeypox: confusion with chickenpox. Acta Trop. 1988 Dec;45(4):297-307. PMID: 2907258.
- Hoff NA, Morier DS, Kisalu NK, Johnston SC, Doshi RH, Hensley LE, Okitolonda-Wemakoy E, Muyembe-Tamfum JJ, Lloyd-Smith JO, Rimoin AW. Varicella Coinfection in Patients with Active Monkeypox in the Democratic Republic of the Congo. Ecohealth. 2017 Sep;14(3):564-574. doi: 10.1007/s10393-017-1266-5. Epub 2017 Sep 11. PMID: 28894977.
- MacNeil A, Reynolds MG, Carroll DS, Karem K, Braden Z, Lash R, Moundeli A, Mombouli JV, Jumaan AO, Schmid DS, Damon IK. Monkeypox or varicella? Lessons from a rash outbreak investigation in the Republic of the Congo. Am J Trop Med Hyg. 2009 Apr;80(4):503-7. PMID: 19346366.
- Reynolds MG, McCollum AM, Nguete B, Shongo Lushima R, Petersen BW. Improving the Care and Treatment of Monkeypox Patients in Low-Resource Settings: Applying Evidence from Contemporary Biomedical and Smallpox Biodefense Research. Viruses. 2017 Dec 12;9(12):380. doi: 10.3390/v9120380. PMID: 29231870; PMCID: PMC5744154.
- World health organisation (WHO). Multi-country Monkey pox outbreak: situation update, 17 June 2022. WHO https://www.who.int/emergencies/disease-outbreak-news/item/2022-DON393.
- European Centre for Disease Prevention and Control (ECDC). Monkey pox multi-country outbreak. Situation update, 22 June 2022. Stockholm: ECDC. https://www.ecdc.europa.eu/en/Monkey pox-outbreak
- World health network (WHN), The WHN declares Monkey pox a pandemic. 28 June 2022. https://www.worldhealthnetwork.global/.
- Beer EM, Rao VB. A systematic review of the epidemiology of human monkeypox outbreaks and implications for outbreak strategy. PLoS Negl Trop Dis. 2019 Oct 16;13(10):e0007791. doi: 10.1371/journal.pntd.0007791. PMID: 31618206; PMCID: PMC6816577.
- Reed KD, Melski JW, Graham MB, Regnery RL, Sotir MJ, Wegner MV, Kazmierczak JJ, Stratman EJ, Li Y, Fairley JA, Swain GR, Olson VA, Sargent EK, Kehl SC, Frace MA, Kline R, Foldy SL, Davis JP, Damon IK. The detection of monkeypox in humans in the Western Hemisphere. N Engl J Med. 2004 Jan 22;350(4):342-50. doi: 10.1056/NEJMoa032299. PMID: 14736926.
- Damon IK. Status of human monkeypox: clinical disease, epidemiology and research. Vaccine. 2011 Dec 30;29 Suppl 4:D54-9. doi: 10.1016/j.vaccine.2011.04.014. Epub 2011 Dec 18. PMID: 22185831.
- Miura F, van Ewijk CE, Backer JA, Xiridou M, Franz E, Op de Coul E, Brandwagt D, van Cleef B, van Rijckevorsel G, Swaan C, van den Hof S, Wallinga J. Estimated incubation period for monkeypox cases confirmed in the Netherlands, May 2022. Euro Surveill. 2022 Jun;27(24):2200448. doi: 10.2807/1560-7917.ES.2022.27.24.2200448. PMID: 35713026; PMCID: PMC9205160.
- Pal M, Singh R, Gutama KP, Savalia CV, Thakur R. Human Monkey pox: An emerging and re-emerging infectious viral disease. Acta Scientific Microbiology. 2022 Apr;5(4).
- Huhn GD, Bauer AM, Yorita K, Graham MB, Sejvar J, Likos A, Damon IK, Reynolds MG, Kuehnert MJ. Clinical characteristics of human monkeypox, and risk factors for severe disease. Clin Infect Dis. 2005 Dec 15;41(12):1742-51. doi: 10.1086/498115. Epub 2005 Nov 11. PMID: 16288398.
- Islam MR, Hossain MJ, Roy A, Hasan AHMN, Rahman MA, Shahriar M, Bhuiyan MA. Repositioning potentials of smallpox vaccines and antiviral agents in monkeypox outbreak: A rapid review on comparative benefits and risks. Health Sci Rep. 2022 Aug 23;5(5):e798. doi: 10.1002/hsr2.798. Erratum in: Health Sci Rep. 2022 Nov 01;5(6):e897. PMID: 36032515; PMCID: PMC9399446.
- Nalca A, Rimoin AW, Bavari S, Whitehouse CA. Reemergence of monkeypox: prevalence, diagnostics, and countermeasures. Clin Infect Dis. 2005 Dec 15;41(12):1765-71. doi: 10.1086/498155. Epub 2005 Nov 11. PMID: 16288402.
- Reynolds MG, McCollum AM, Nguete B, Shongo Lushima R, Petersen BW. Improving the Care and Treatment of Monkeypox Patients in Low-Resource Settings: Applying Evidence from Contemporary Biomedical and Smallpox Biodefense Research. Viruses. 2017 Dec 12;9(12):380. doi: 10.3390/v9120380. PMID: 29231870; PMCID: PMC5744154.
- Guagliardo SAJ, Monroe B, Moundjoa C, Athanase A, Okpu G, Burgado J, Townsend MB, Satheshkumar PS, Epperson S, Doty JB, Reynolds MG, Dibongue E, Etoundi GA, Mathieu E, McCollum AM. Asymptomatic Orthopoxvirus Circulation in Humans in the Wake of a Monkeypox Outbreak among Chimpanzees in Cameroon. Am J Trop Med Hyg. 2020 Jan;102(1):206-212. doi: 10.4269/ajtmh.19-0467. PMID: 31769389; PMCID: PMC6947779.
- Adler H, Gould S, Hine P, Snell LB, Wong W, Houlihan CF, Osborne JC, Rampling T, Beadsworth MB, Duncan CJ, Dunning J, Fletcher TE, Hunter ER, Jacobs M, Khoo SH, Newsholme W, Porter D, Porter RJ, Ratcliffe L, Schmid ML, Semple MG, Tunbridge AJ, Wingfield T, Price NM; NHS England High Consequence Infectious Diseases (Airborne) Network. Clinical features and management of human monkeypox: a retrospective observational study in the UK. Lancet Infect Dis. 2022 Aug;22(8):1153-1162. doi: 10.1016/S1473-3099(22)00228-6. Epub 2022 May 24. Erratum in: Lancet Infect Dis. 2022 Jul;22(7):e177. Erratum in: Lancet Infect Dis. 2022 Jul;22(7):e177. PMID: 35623380; PMCID: PMC9300470..
- Russo AT, Grosenbach DW, Brasel TL, Baker RO, Cawthon AG, Reynolds E, Bailey T, Kuehl PJ, Sugita V, Agans K, Hruby DE. Effects of Treatment Delay on Efficacy of Tecovirimat Following Lethal Aerosol Monkeypox Virus Challenge in Cynomolgus Macaques. J Infect Dis. 2018 Sep 22;218(9):1490-1499. doi: 10.1093/infdis/jiy326. PMID: 29982575; PMCID: PMC6151088.
- Hoy SM. Tecovirimat: First Global Approval. Drugs. 2018 Sep;78(13):1377-1382. doi: 10.1007/s40265-018-0967-6. PMID: 30120738.
- Vivancos R, Anderson C, Blomquist P, Balasegaram S, Bell A, Bishop L, Brown CS, Chow Y, Edeghere O, Florence I, Logan S, Manley P, Crowe W, McAuley A, Shankar AG, Mora-Peris B, Paranthaman K, Prochazka M, Ryan C, Simons D, Vipond R, Byers C, Watkins NA; UKHSA Monkeypox Incident Management team; Welfare W, Whittaker E, Dewsnap C, Wilson A, Young Y, Chand M, Riley S, Hopkins S; Monkeypox Incident Management Team. Community transmission of monkeypox in the United Kingdom, April to May 2022. Euro Surveill. 2022 Jun;27(22):2200422. doi: 10.2807/1560-7917.ES.2022.27.22.2200422. Erratum in: Euro Surveill. 2022 Jun;27(23): PMID: 35656834; PMCID: PMC9164677.
- Mauldin MR, McCollum AM, Nakazawa YJ, Mandra A, Whitehouse ER, Davidson W, Zhao H, Gao J, Li Y, Doty J, Yinka-Ogunleye A, Akinpelu A, Aruna O, Naidoo D, Lewandowski K, Afrough B, Graham V, Aarons E, Hewson R, Vipond R, Dunning J, Chand M, Brown C, Cohen-Gihon I, Erez N, Shifman O, Israeli O, Sharon M, Schwartz E, Beth-Din A, Zvi A, Mak TM, Ng YK, Cui L, Lin RTP, Olson VA, Brooks T, Paran N, Ihekweazu C, Reynolds MG. Exportation of Monkeypox Virus From the African Continent. J Infect Dis. 2022 Apr 19;225(8):1367-1376. doi: 10.1093/infdis/jiaa559. PMID: 32880628; PMCID: PMC9016419.