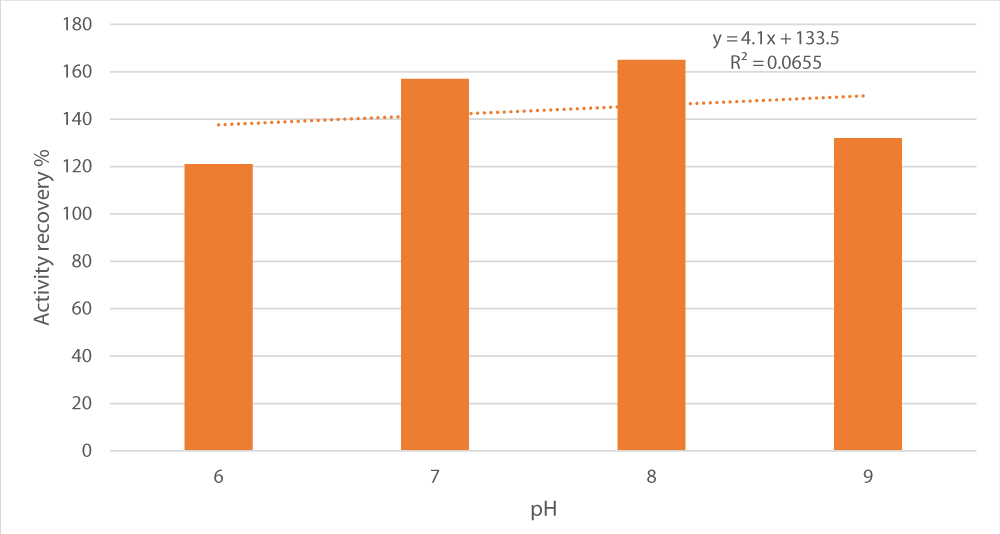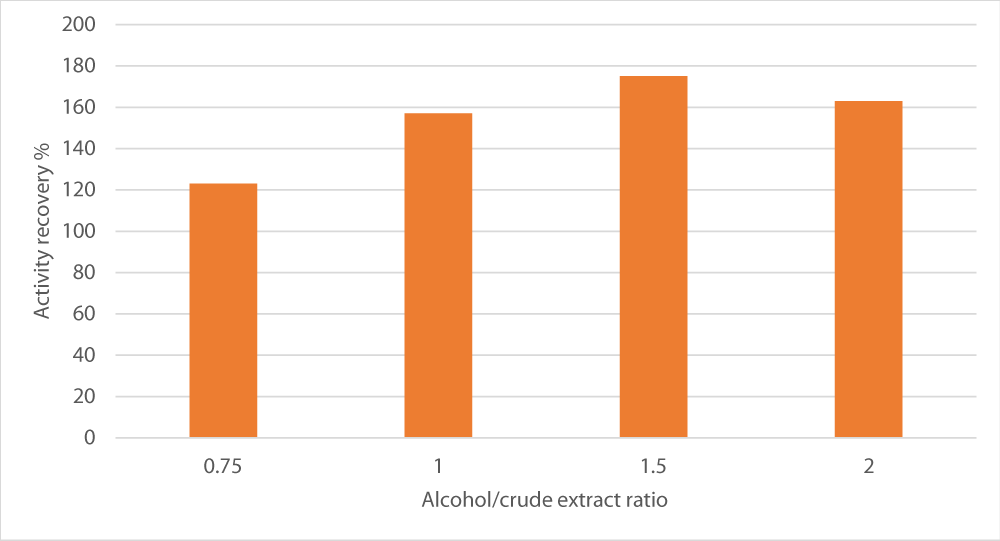More Information
Submitted: March 14, 2023 | Approved: March 20, 2023 | Published: March 21, 2023
How to cite this article: Al-Madhagi HA, Yazbik V, Abdelwahed W. Three-phase partitioning for isolating peroxidase from lemon peels. Ann Proteom Bioinform. 2023; 7: 001-005.
DOI: 10.29328/journal.apb.1001018
Copyright License: © 2023 Al-Madhagi HA, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Keywords: Peroxidase; Three-phase partitioning; Isolation; Activity recovery; Enzyme
Three-phase partitioning for isolating peroxidase from lemon peels
Haitham Ahmed Al-Madhagi1* Valantina Yazbik1 and Wassim Abdelwahed3
Valantina Yazbik1 and Wassim Abdelwahed3
1Department of Chemistry, Faculty of Science, University of Aleppo, Syria
2Biochemical Technology Program, Dhamar University, Yemen
3Department of pharmaceutics, Faculty of pharmacy, Aleppo University, Aleppo, Syria
*Address for Correspondence: Haitham Ahmed Al-Madhagi, Department of Chemistry, Faculty of Science, University of Aleppo, Syria, Biochemical Technology Program, Dhamar University, Yemen, Email: [email protected]
Background and objective: Peroxidase is an oxidoreductase that uses different compounds as substrates and thus can be utilized for different applications. The goal of this work is to isolate peroxidase from lemon peels using Three-Phase Portioning (TPP).
Methods: TPP was set by adding varying amounts of salts and alcohol and the enzyme activity recovery was measured for each variable. Different parameters were optimized successively in order to achieve the highest enzyme activity recovery including salt type, salt concentration, pH, alcohol/crude extract ratio and type of alcohol and then, combining all optimized conditions together.
Results: Salt that gave maximal recovery was sodium potassium tartrate, optimal salt concentration was 15%, optimal pH was 8, optimal alcohol/crude extract ratio was 1 and t-butanol was preferred to 1-butanol. Efficiently, upon combining all optimized factors, an activity recovery of 175% was obtained.
Conclusion: This protocol provides an easy, feasible method to efficiently isolate peroxidase from lemon peels using TPP.
Enzymes are highly efficient protein catalyst that speeds up various chemical reactions. Based on the reaction they catalyze, enzymes can be classified into six groups, namely oxidoreductase, transferases, hydrolases, lyases, isomerases and ligases. Under each class there are subclasses. Peroxidase enzyme belongs to oxidoreductase [1]. Being oxidoreductases, peroxidases (E.C. 1.11.1.X) oxidize a wide spectrum of substrates by decomposing hydrogen peroxide. The substrates peroxidase can catalyze include aliphatic, aromatic, organic and inorganic. Peroxidase is distributed almost throughout all life kingdoms from bacteria to humans but dominates in plants [2]. This type of enzyme differs in its primary as well as the tertiary structure since the molecular weight ranges from 30 kDa to 150 kDa. Due to the identification, characterization and application of this enzyme from several sources for a plethora of purposes, a database was established to contain such information called PeroxiBase [3].
Peroxidase can be used for different purposes involving the food industry such as milk sterilization, environmental remediation such as dyes and mycotoxin degradation, chemical syntheses like purpurogallin and as a biosensor in the diagnostic kits of glucose and triglycerides, among others. Such a wide application spectrum is attributed to the wide acceptability of the enzyme toward plenty of substrates whatever the source [4].
The first step prior to application is enzyme isolation. The most commercial, commonly used source of peroxidase isolation is horseradish [5]. Nevertheless, the commercial source is not usually feasible owing to economic cost issues. In contrast, new sources were employed for the isolation of peroxidase and demonstrated their replaceability with horseradish peroxidase [6]. Three-Phase Portioning (TPP) is a non-chromatographic, rapid, easy-to-use method for the isolation and purification of various biomolecules including, bioactive phenolic compounds, polysaccharides, enzymes, proteins and drugs. As its name implies, it is composed of three phases but with two components, i.e. upper alcohol phase (dominantly tert-butanol), middle phase (dominantly insoluble protein clumps) and lower aqueous phase (saturated with salt). The fractionation principle is based on the affinity of the fractioned molecules to each phase of the system. Of note, TPP has payed attention in the recent years due to high recovery yield of the isolated biomolecule and can be coupled with ultrasound and microwave [7].
Herein, we aimed to isolate peroxidase from lemon peels using TPP.
Reagents
Sodium dihydrogen phosphate, disodium hydrogen phosphate, ammonium sulfate and potassium sodium tartrate were purchased from Merck whereas sodium citrate, sodium acetate and t-butanol were purchased from HiMedia. The phosphate buffer used was prepared at pH 7 and 100 mM concentrations.
Preparation of lemon peels
Fresh lemon was purchased from the local market of Aleppo city, Syria. About 3 mm was the thickness of the excised rind that was cut from the washed watermelon which was further washed twice with distilled water. Then, 10 g of the rinds together with 100 ml of cold phosphate buffer (pH 7) were homogenized using a commercial blender. Afterward, the obtained solution was filtered and centrifuged at 5500 rpm for 20 min and the supernatant was stored at 4 °C for later use.
TPP setup
In a 15 ml test tube, 5 ml of the crude extract was saturated with different salt concentrations and mixed until completely dissolved. Then, various volumes of alcohol were added to the tube and vigorously mixed and settled for 3 h. After that, a brief centrifugation step was done to facilitate the separation of the layers (3000 rpm for 5 min). The enzymatic activity was measured for the bottom layer. However, many parameters were optimized to achieve maximum separation efficiency involving different salt types, salt concentrations, pH degrees, different alcohol types and varying the alcohol/crude extract ratios [6].
Determination of peroxidase assay
We followed the methods described by [8]. In short, 2.4 ml of phosphate buffer (pH 7) was added to a test tube followed by the addition of 300 µl 5.3% pyrogallol, 200 µl 0.6% hydrogen peroxide and 100 µl of the corresponding enzyme source at 420 nm for 4 min. The activity recovery was calculated by dividing the obtained activity by the activity of the crude extract multiplied by 100.
Determination of optimum pH and temperature
The isolated peroxidase was analyzed for the determination of its optimal pH and temperature. Different pH values were examined, i.e. from 4 - 9. Similarly, incremental temperatures (15 °C - 85 °C) were tested for the potential influence on the isolated peroxidase.
Statistical analysis
The data obtained in the present study were analyzed using Microsoft Excel® 2019. The same program was utilized for the graphical representation of results as well as the regression analysis.
The measured activity of the crude extract was found to be 824 U/L which instigates us to further isolate the enzyme from such attainable fruit waste. All the factors examined were measured in duplicates.
Effect of salt concentration
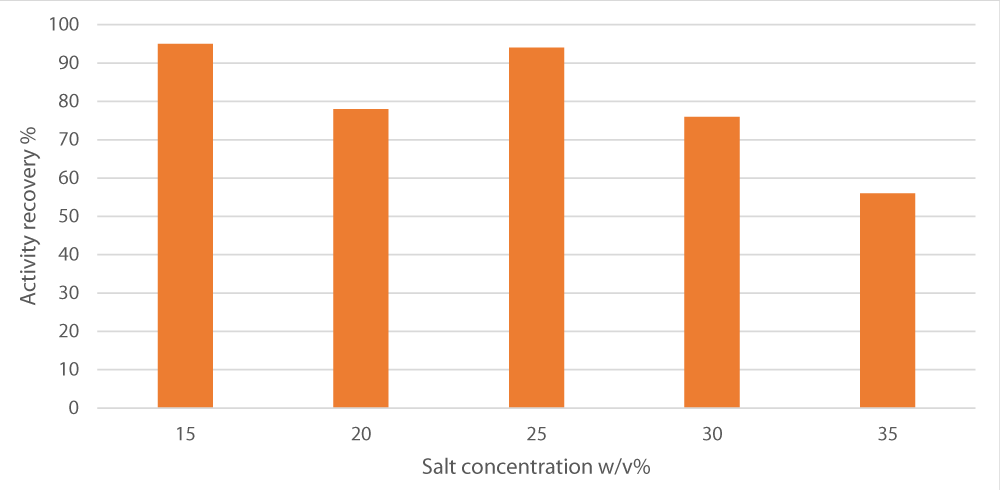
After selecting the best salt source, we optimized sodium potassium tartrate that gives maximum separation by varying the concentration range from 15% to 35%. We found that as the salt concentrations, 15% and 25 w/v posed the highest, separation increases and enzyme activity recovery raises to give maximal activity (95 and 94%). However, after that concentration, raising the salt concentration poses a negative impact on the separation process as illustrated in Figure 1.
Figure 1: Effect of varying ammonium sulfate concentration on peroxidase recovery. pH was set at 7, the alcohol/crude extract ratio at 1 and the alcohol used was t-butanol.
Effect of salt type
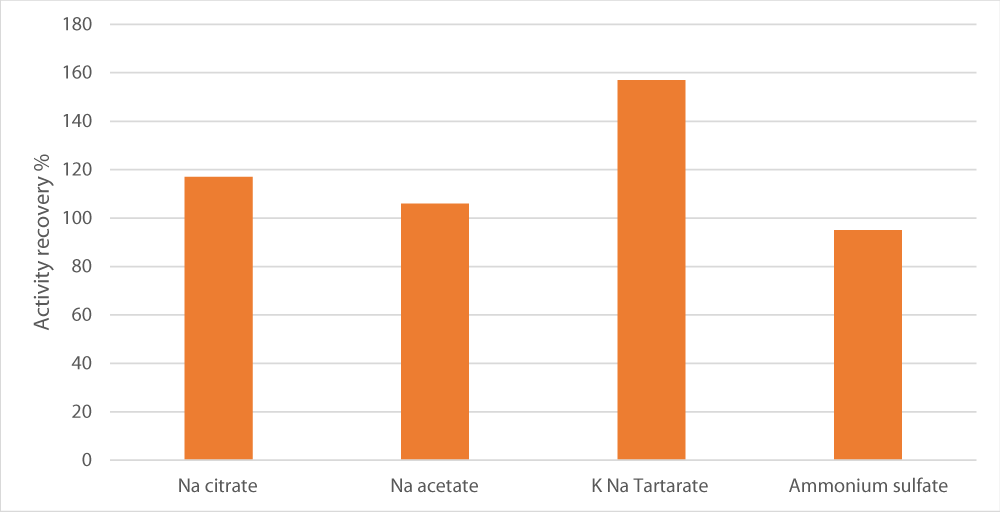
To test the influence of salt type on the separation of peroxidase into the bottom layer, various salts were examined. Among those, sodium potassium tartrate exhibited the highest relative activity (157%) in comparison with the crude extract as depicted in Figure 2. This was followed by magnesium sulfate and sodium acetate with activity recovery of 117%.
Figure 1: Effect of the salt type used to saturate aqueous phase on enzyme recovery. Salt concentration was set at 15%, pH at 7, alcohol/crude extract ratio at 1 and the alcohol used was t-butanol.
Effect of pH
Next, we tested the impact of pH variation (pH 6 to 9) on peroxidase recovery (Figure 3). A bell curve was obtained with peak recovery (165%) at pH 8 making it the optimal pH for peroxidase fractionation. Noticeably, the coefficient of variance (R2) of pH and activity recovery association was 0.0655, indicating there was no relationship between them statistically.
Figure 3: Influence of pH on peroxidase recovery. Salt concentration was set at 15%, the salt used was sodium potassium tartrate, the alcohol/crude extract ratio at 1 and the alcohol used was t-butanol.
Effect of alcohol type
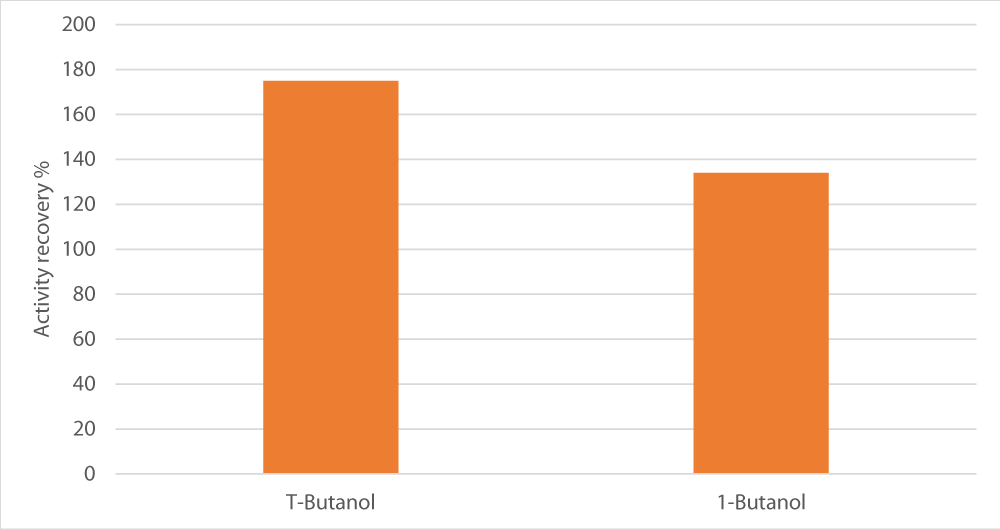
We also tested 1-butanol versus the standard alcohol used in TPP, T-butanol. Indeed, t-butanol gave better findings than 1-butanol, i.e. 175% versus 134% of activity recovery, respectively as shown in Figure 4.
Figure 4: Influence of type of alcohol used on enzyme recovery. The salt used was sodium potassium tartrate, salt concentration was set at 15%, pH at 8 and alcohol/crude extract ratio at 1.5.
Effect of alcohol/crude extract ratio
Finally, the last parameter to be optimized was the ratio between alcohol and crude extract in terms of volumes on the fractionation. A sharp increase was observed in the ration 1.5, the fractionation was maximal (175%). However, below and beyond this ratio, peroxidase recovery was reduced (Figure 5).
Figure 5: Effect of alcohol/crude extract ratio on peroxidase recovery. The salt used was sodium potassium tartrate, salt concentration was set at 15% and pH at 8.
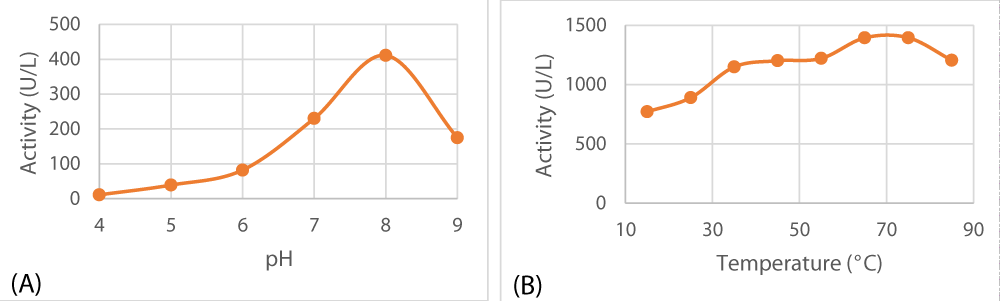
Optimum pH and temperature
The isolated peroxidase tends to favor a slightly alkaline medium for optimal activity (pH 8). With respect to temperature, it turned out that it is an exceptionally thermostable enzyme that can tolerate temperatures as hot as 75 °C as depicted in Figure 6.
Figure 6: Optimal pH (A) and optimal temperature (B) of lemon peel peroxidase.
Agricultural wastes encompass a large family of residual materials generated during of after agricultural activities like coffee pulp from the coffee industry, husks from the cereal industry and peels from the starch-based industry [9]. Indeed, huge amounts of these wastes are produced every year which necessitate downstream processing for simultaneous valorization of such wastes and eliminating the concomitating adverse effects. Agro-industrial wastes are used for manufacturing of biofuels, enzymes, vitamins, antioxidants, animal feed, antibiotics and other chemicals [10].
TPP is an efficient, cost-effective and easy-to-use bioseparation technique which has proven its efficacy in terms of high yield of isolated biomolecules, mainly enzymes, from crude extracts. TPP has many advantages as a fractionation method such as requirement of mild conditions, recycling of chemicals used, less time demanding as well as being a fast approach [11]. Furthermore, TPP is very effective in fractioning crude extract components, i.e. lipids and nonpolar substances move to t-butanol layer while some proteins are aggregating at the interface layer. This in turn leads to the significant recovery of the desired enzyme [12]. Indeed, several enzymes were isolated from many sources and wastes using TPP including lipase [13], bromelain [14], papain [15], and other proteases for enhanced activity [16].
Upon combining the optimized parameters, namely, using sodium potassium tartrate as the salt source, with a concentration 15%, at pH 8, alcohol to crude extract ratio of 1.5 and t-butanol as the alcohol source, approximately 175% of the separated enzyme activity was obtained in the bottom phase while other proteins and phenolic compounds were aggregated in the middle and upper layers respectively. This indicates that almost all of the enzyme was transferred into the lower layer reflecting the high separation efficiency. In addition, the extracting salt (sodium potassium tartrate) had a slight activating action of peroxidase activity which account for the extra in the percentage over 100%. Increasing the ration between alcohol and crude extract resulted in removing the inhibitors of the enzyme from the aqueous phase [17]. Given the fact that the isolated peroxidase can withstand harsh conditions of pH (weakly alkaline pH of and temperatures (up to 75 °C), this makes it superior in the field of industrial applications that necessitates such powerful enzymes [18]. TPP was previously utilized to isolate and purify peroxidase from different sources. Vetal and Rathod [19] upon applying TPP for peroxidase isolation from orange peels, the activity recovery of the enzyme obtained was 93% after 180 min. Karakus, et al. [8] purified peroxidase enzyme from Amsonia orientalis plant using TPP. The activity recovery was after 30 min 162%. Moreover, TPP can be coupled with ultrasonication to further reduce the time of fractionation. This approach was employed to purify peroxidase enzyme from orange peels with activity recovery 91% within 6 min [20]. Nonetheless, this technique had a negative effect on the activity recovery of our study (diminished to 76%).
This study demonstrates the utilization of TPP to isolate peroxidase enzyme from a waste material (lemon peels). Successive optimizations were performed so as to harvest the highest activity recovery of peroxidase. Salt that gave maximal recovery was sodium potassium tartrate, optimal salt concentration was 15%, optimal pH was 8, optimal alcohol/crude extract ratio was 1.5 and t-butanol was preferred to 1-butanol. Indeed, upon combining all optimized factors, activity recovery of 175% was obtained reflecting the high yield of enzyme isolation as well as the feasibility and efficiency of the TPP as a method of isolation. We recommend to purify peroxidase from other waste materials using the same approach and examine potential applications of the isolated enzyme.
- Nelson DA, Cox M. Lehninger Principles of Biochemistry: International Edition. 2016.
- Demarche P, Junghanns C, Nair RR, Agathos SN. Harnessing the power of enzymes for environmental stewardship. Biotechnol Adv. 2012 Sep-Oct;30(5):933-53. doi: 10.1016/j.biotechadv.2011.05.013. Epub 2011 May 20. PMID: 21624453.
- Passardi F, Theiler G, Zamocky M, Cosio C, Rouhier N, Teixera F, Margis-Pinheiro M, Ioannidis V, Penel C, Falquet L, Dunand C. PeroxiBase: the peroxidase database. Phytochemistry. 2007 Jun;68(12):1605-11. doi: 10.1016/j.phytochem.2007.04.005. Epub 2007 Jun 4. PMID: 17544465.
- de Oliveira FK, Santos LO, Buffon JG. Mechanism of action, sources, and application of peroxidases. Food Res Int. 2021 May; 143:110266. doi: 10.1016/j.foodres.2021.110266. Epub 2021 Mar 5. PMID: 33992367.
- Liu J-Z, Wang T-L, Ji L-N. Enhanced dye decolorization efficiency by citraconic anhydride-modified horseradish peroxidase. J Mol Catal B Enzym 2006; 41:81–6.
- Narayan AV, Madhusudhan MC, Raghavarao KS. Extraction and purification of Ipomoea peroxidase employing three-phase partitioning. Appl Biochem Biotechnol. 2008 Dec;151(2-3):263-72. doi: 10.1007/s12010-008-8185-4. Epub 2008 Mar 28. PMID: 18369532.
- Chew KW, Ling TC, Show PL. Recent developments and applications of three-phase partitioning for the recovery of proteins. Sep Purif Rev 2019; 48:52–64.
- Yuzugullu Karakus Y, Acemi A, Işık S, Duman Y. Purification of peroxidase from Amsonia orientalis by three-phase partitioning and its biochemical characterization. Sep Sci Technol 2018; 53:756–66. https://doi.org/10.1080/01496395.2017.1405990.
- Sadh PK, Duhan S, Duhan JS. Agro-industrial wastes and their utilization using solid state fermentation: a review. Bioresour Bioprocess 2018; 5:1. https://doi.org/10.1186/s40643-017-0187-z.
- Yusree FIFM, Peter AP, Mohd Nor MZ, Show PL, Mokhtar MN. Latest Advances in Protein-Recovery Technologies from Agricultural Waste. Foods. 2021 Nov 9;10(11):2748. doi: 10.3390/foods10112748. PMID: 34829028; PMCID: PMC8618363.
- Vidhate GS, Singhal RS. Extraction of cocoa butter alternative from kokum (Garcinia indica) kernel by three phase partitioning. J Food Eng 2013; 117:464–6.
- Ketnawa S, Rungraeng N, Rawdkuen S. Phase partitioning for enzyme separation: An overview and recent applications. Int Food Res J 2017; 24:1.
- Kublicki M, Koszelewski D, Brodzka A, Ostaszewski R. Wheat germ lipase: isolation, purification and applications. Crit Rev Biotechnol. 2022 Mar;42(2):184-200. doi: 10.1080/07388551.2021.1939259. Epub 2021 Jul 15. PMID: 34266327.
- Gul A, Khan S, Arain H, Khan H, Ishrat U, Siddiqui M. Three-phase partitioning as an efficient one-step method for the extraction and purification of bromelain from pineapple crown waste. J Food Process Preserv 2022; 46:e16973. https://doi.org/10.1111/jfpp.16973.
- Jain J. Review on isolation and purification of papain enzyme from papaya fruit. Int J Eng Appl Sci Technol 2020; 5:193–7.
- Eyssen LE, Goldring JPD, Coetzer THT. Three-phase partitioning (TPP) of proteases from parasites, plants, tissue and bacteria for enhanced activity. Three Phase Partitioning, Elsevier. 2021; 133–54.
- Gagaoua M, Hafid K. Three phase partitioning system, an emerging non-chromatographic tool for proteolytic enzymes recovery and purification. Biosens J 2016; 5:100134.
- Herlet J, Kornberger P, Roessler B, Glanz J, Schwarz WH, Liebl W, Zverlov VV. A new method to evaluate temperature vs. pH activity profiles for biotechnological relevant enzymes. Biotechnol Biofuels. 2017 Oct 11; 10:234. doi: 10.1186/s13068-017-0923-9. PMID: 29046720; PMCID: PMC5637330.
- Vetal MD, Rathod VK. Three phase partitioning a novel technique for purification of peroxidase from orange peels (Citrus sinenses). Food Bioprod Process 2015; 94:284–9. https://doi.org/10.1016/j.fbp.2014.03.007.
- Vetal MD, Rathod VK. Ultrasound assisted three phase partitioning of peroxidase from waste orange peels. Green Process Synth 2016; 5:205–12. https://doi.org/10.1515/gps-2015-0116.